What Will Happen to the Rate of Photosynthesis if the Size of the Stomata Is Increased?
Introduction
Enhancing leafage photosynthesis has been attempted to drive further increases in biomass product in ingather plants (von Caemmerer and Evans, 2010; Yamori et al., 2016; Sakoda et al., 2018). Gas diffusional resistance from the temper to the chloroplast is one of the limiting factors for leaf photosynthetic capacity (Farquhar and Sharkey, 1982). Stomata, pores on the epidermis of plant leaves, function to maintain the residue between CO2 uptake for photosynthesis and water loss for transpiration (Mcadam and Brodribb, 2012). It has been highlighted that the conductance to gas diffusion via stomata (chiliad s ) can be a major determinant of COtwo assimilation rate (A) (Wong et al., 1979). The potential of g s is mainly determined by the size, depth, and opening of unmarried stoma, and their density (Franks and Beerling, 2009). Information technology has been controversial how the change in the stomatal density (SD), defined as the stomata number per unit leaf area, affects photosynthetic and growth characteristics in plants (Lawson and Blatt, 2014). Doheny-Adams et al. (2012) reported that lower SD yielded higher growth charge per unit and biomass production in Arabidopsis under constant lite owing to the favorable water condition and temperature for metabolism and depression metabolic cost for stomatal development (Doheny-Adams et al., 2012). Contrastingly, lower SD resulted in the depression of thousand s and/or A in Arabidopsis and poplar plants (Büssis et al., 2006; Yoo et al., 2010; Wang et al., 2016). An SDD1 knockout line of Arabidopsis with higher SD showed higher g s and A than a wild-blazon line, depending on calorie-free condition (Schlüter et al., 2003). Previously, we reported that higher SD past overexpressing STOMAGEN/EPFL9 resulted in the enhancement of chiliad s and A in Arabidopsis under abiding and high light conditions (Tanaka et al., 2013). Therefore, SD manipulation could accept the potential to enhance photosynthetic and growth characteristics in plants, even though that effect can depend on the species or environmental weather.
In the field, calorie-free intensity tin can fluctuate at different scales, from less seconds to minutes, over the course of a day owing to changes in the solar radiation, cloud cover, or self-shading in the plant canopy (Kaiser et al., 2018). The gradual increment in A can be shown later the transition from low to high light intensity, and this phenomenon is called "photosynthetic induction." A simulation analysis demonstrated that the potential loss of the cumulative corporeality of CO2 assimilation caused by photosynthetic consecration tin reach at least 21% in wheat (Triticum aestivum L.) and soybean (Glycine max (L.) Merr.) (Taylor and Long, 2017; Tanaka et al., 2019). In rice (Oryza sativa 50.) and soybean, there is genotypic variation in the speed of photosynthetic consecration, which causes pregnant differences in the cumulative carbon gain under fluctuating light (Soleh et al., 2016, 2017; Adachi et al., 2019). Consequently, speeding up photosynthetic induction can yield more efficient carbon proceeds, which will open up a new pathway to better biomass production in plants under field conditions.
Photosynthetic consecration is typically limited by three phases of the biochemical and diffusional processes: (1) activation of electron send, (2) activation of the enzymes in the Calvin-Benson wheel, and (three) stomatal opening (Pearcy, 1990; Yamori, 2016; Yamori et al., 2020). Especially, the activation of Rubisco (5–10 min for full induction) and stomatal opening (20–30 min for total induction) constitute a major limitation to photosynthetic induction (Yamori et al., 2012; Carmo-Silva and Salvucci, 2013). The overexpression of PATROL1, controlling the translocation of a major H+-ATPase (AHA1) to the plasma membrane, resulted in faster g due south induction to fluctuating low-cal in Arabidopsis without the change in SD (Hashimoto-Sugimoto et al., 2013). Arabidopsis knockout mutants of ABA transporter, which plays pivotal roles in stomatal closure, improved stomatal response to fluctuating calorie-free and photosynthesis (Shimadzu et al., 2019). Furthermore, the rapid stomatal response is important for plants to achieve loftier water apply efficiency (WUE) (Qu et al., 2016). Notably, the faster stomatal opening improved the photosynthetic induction and thus biomass production in Arabidopsis nether the fluctuating low-cal (Papanatsiou et al., 2019; Kimura et al., 2020). These facts evidence that rapid stomatal response can be benign for the effective carbon gain and water use under fluctuating light weather condition. However, how SD changes affect g s and A dynamics, biomass production, and water use under these conditions has been understudied (Drake et al., 2013; Papanatsiou et al., 2016; Schuler et al., 2017; Vialet-Chabrand et al., 2017).
Information technology is hypothesized that higher SD results in college initial g s (Tanaka et al., 2013), which can contribute to faster photosynthetic induction due to the lower stomatal limitation nether the fluctuating light. The objective of this report was to examine how higher SD affects the photosynthetic and growth characteristics in plants nether fluctuating calorie-free conditions. Hither, nosotros investigated the induction response of g south , A, transpiration charge per unit (East) and water use efficiency (WUE) subsequently step increase in lite by gas exchange measurements, and biomass product under fluctuating light conditions in the three Arabidopsis lines differing in SD.
Materials and Methods
Plant Materials and Growth Conditions
The peptide signals in a family of EPIDERMAL PATTERNING Factor (EPF) were identified to function in the stomatal evolution of Arabidopsis (Arabidopsis thaliana (L.) Heynh) (Hara et al., 2007). It has been demonstrated that EPF1 and EPF2 combine with the receptor-like protein, TOO MANY MOUTHS (TMM) and ERECTA family leucine-rich repeat-receptor-similar kinases and, consequently, restrain a specific process in stomatal development. Contrastingly, STOMAGEN/EPFL9 combines with TMM competitively to EPF1 and EPF2, and promote stomatal development (Sugano et al., 2010; Lee et al., 2015). In the nowadays study, Columbia-0 (CS60000) of Arabidopsis thaliana (L.) Heynh, was used as a wild-blazon line (WT). In addition, we used STOMAGEN/EPFL9 overexpressing line (ST-OX10-3; ST-OX) which was used in Tanaka et al. (2013), and an EPF1 knockout line (SALK_137549) (epf1-1; epf1) which was used in Sugano et al. (2010).
For analyzing photosynthetic and stomatal traits, 6 plants per line were sown and grown in the soil in the growth chamber at an air humidity of 60%, COtwo concentration of 400 μmol mol–ane and a photosynthetic photon flux density (PPFD) of 100 μmol photon grand–2 southward–1 for the gas exchange analysis. The twenty-four hour period/night period was set to 8/sixteen h with a abiding air temperature of 22°C. We randomly inverse establish organisation every iii–4 days during their growth period to avoid the spacing effects. For the biomass analysis, plants were sown and grown in the soil at an air temperature of 22°C and a PPFD of 120 μmol photon m–ii s–one for 24 days subsequently sowing with the day/night period of 8/xvi h. Later on, four plants per line were subjected to constant and fluctuating light weather, for 20 days with a 24-hour interval/nighttime cycle of 12/12 h. During daytime, the light intensity in the abiding light condition was changed from a PPFD of 60 μmol photon 1000–two south–1 for four h to 500 μmol photon m–two s–1 for 4 h, followed by threescore μmol photon yard–2 southward–1 for 4 h, while a PPFD of 60 μmol photon thou–2 due south–1 for 10 min after 500 μmol photon m–2 s–one for 5 min was repeated for 12 h in the fluctuating lite condition every bit described in Kimura et al. (2020). Plants were exposed to the same total amount of calorie-free intensity per 24-hour interval nether both light conditions. Nosotros randomly inverse plant arrangement every 3–4 days during their growth period to avoid the spacing effects. Dry weight of above ground biomass grown under each lite condition was evaluated at 44 days later sowing.
Evaluation of Stomatal Density, Size, and Clustering
The stomatal density (SD), size (L g ), and clustering were evaluated in the leaves of the six plants per line at the same growth stage as the gas exchange measurements were conducted. We used the six leaves of the three plants in which gas exchange measurements were conducted and the other three plants. A section of the leaf (five × v mm) was excised and immediately stock-still in the solution (Ethanol : acetic acrid = 9:ane, five/v) overnight. The fixed tissues were cleared in chloral hydrate solution (chloral hydrate : glycerol : water = 8:ane:2, w/v/v) overnight. The cleared tissues were stained with safranin-O solution (200 μg ml–1) for 30 min to 1 h. The abaxial side of the leaves was observed at a 200 × magnification using an optical microscope and six digital images (0.072 mm2) were obtained per foliage (CX31 and DP21, Olympus, Tokyo, Japan). We used imaging assay software, ImageJ (NIH, Bethesda, MD, United states) to appraise the stomatal number and guard cell length from the images. SD was calculated from the stomatal number per unit of measurement leafage area. L g , divers equally baby-sit cell length, of all the stomata (2–86 stomata) was measured in each image. Each clustering category (2–five er) means the number of clustered stomata. The percentage of clustered stomata to full number was measured for each clustering category from 2 to 5 as described in Hara et al. (2007). The hateful values of each trait were calculated in six images obtained from each leaf. Subsequently, the boilerplate value of each trait for six leaves was calculated for each line.
Gas Commutation Measurements
Gas exchange measurements were conducted using a portable gas-commutation system LI-6400 (LI-COR, Lincoln, NE, United states of america). All plants were kept in the dark (a PPFD of 0 μmol photon g–2 s–1) overnight before and during the measurements. In the foliage bedroom, we gear up flow rate at 300 μmol s–1, COtwo concentration at 400 μmol mol–1, and air temperature at 25°C. After the leafage was clamped in the chamber, low-cal intensity was kept at a PPFD of 0 μmol photon g–2 s–1 for the initial ten min and, subsequently, under a PPFD of 500 μmol photon k–2 s–ane for 120 min. A, g s , intercellular COii concentration (C i ), and Due east were recorded every ten s during the measurements. WUE was calculated as the ratio of A to Eastward. Gas exchange measurements were conducted with iii plants per line during 68 to 73 days after sowing.
Data Processing
To evaluate the induction speeds of A and g s , we calculated A consecration and 1000 s induction defined equally the following equations:
where A i and g si represent steady-state values under a PPFD of 0 μmol photon thou–2 due south–1, steady-state A and g south , A f and one thousand s f , represent the maximum values which were reached in 120 min nether a PPFD of 500 μmol photon m–2 s–1, and A t and g s t represent values at a given time under illumination. We evaluated the differences in the time when A consecration and g sinduction reached the closest values of 5, 10, xx, xl, 60, and 80% of those maximum values after pace alter in light from 0 to 500 μmol photon 1000–ii s–1 (t v – 80 g south and t five – 80 A ) between WT and ST-OX or epf1.
The cumulative CO2 assimilation (CCA) and transpiration (CE) nether fluctuating lite were calculated by summing A and E in first ten min nether illumination after the initial night period. An integrated WUE (WUE i ) was calculated as the ratio of CCA to CE. Assuming the absence of induction response of A to the pace increase in lite, a theoretically maximum CCA (CCA t ) was defined by the following equation:
where T 500 is the seconds for which the lite intensity was maintained at 500 μmol photon k–2 southward–ane for 10 min. The potential loss charge per unit of CCA caused past photosynthetic consecration was defined by the following equation:
Statistical Analysis
The variation in stomatal size and all the parameters of photosynthetic and growth characteristics were compared between WT and ST-OX or epf1 past a Dunnett'due south examination. Steel test was applied to evaluate SD variation between WT and ST-OX or epf1 considering the distribution of values was extremely dissimilar among the lines. Statistical analysis was conducted using R software version 3. 6. 1 (R Foundation for Statistical Calculating, Vienna, Austria).
Results
Stomatal Density, Size, and Clustering
We evaluated stomatal density (SD), size (50 one thousand ) and clustering in the three Arabidopsis lines. ST-OX and epf1 showed 268.one and 46.v% higher SD than WT (p < 0.05) (Effigy 1A). L g of ST-OX was 10.0% lower than that of WT (p < 0.01) (Figure 1B). Stomatal clustering was scarcely observed in WT, while two to v stomata were clustered in ST-OX and epf1 (Figure 1C). The ratio of stomata in each clustering category was higher in ST-OX than that in epf1.
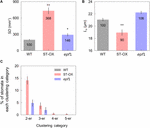
Figure 1. Stomatal density, size, and clustering. (A) The stomatal density (SD), (B) guard jail cell length (L g ) and (C) the charge per unit of stomata in 2–five clustering categories were evaluated on fully expanded leaves in the wild-blazon line (WT), a STOMAGEN/EPFL9 overexpressing line (ST-OX), and an EPF1 knockout line (epf1) of Arabidopsis thaliana. The vertical confined betoken the standard error (n = 6). * and ** indicate the meaning variation in each parameter between WT and each transgenic line at p < 0.05, and 0.01, respectively, according to the Steel test in (A) or Dunnett's exam in (B). The value in each column represents the relative value of each line to WT.
Photosynthesis and Stomatal Conductance After Step Increment in Light
To examine how higher SD affects the photosynthetic characteristics under the fluctuating light, we conducted gas exchange measurements. ST-OX and epf1 maintained higher one thousand due south , C i , A, and Eastward than WT under the non-steady state at high light intensity (500 μmol photon grand–2 s–one) (Figures 2A–D), while they showed lower WUE (Figure 2E). Under the steady state, there was no significant difference in g southward and A between WT and ST-OX or epf1, although these parameters of ST-OX and epf1 tended to be higher than those of WT (Supplementary Effigy S1).
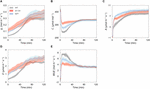
Figure 2. Photosynthetic dynamics afterward footstep increase in light. (A) A stomatal conductance (g due south ), (B) intercellular COtwo concentration (C i ), (C) COtwo assimilation charge per unit (A), (D) transpiration rate (Due east), and (E) water use efficiency (WUE) were measured on fully expanded leaves in the iii lines of Arabidopsis. The gas exchange measurements were conducted at a CO2 concentration of 400 ppm, air temperature of 25°C and night status for the initial 10 min and, later, under a PPFD of 500 μmol photon m–2 due south–ane for 120 min. Vertical confined indicate the standard mistake (northward = iii).
Subsequently, nosotros evaluated the induction speed of chiliad southward and A to the stride increment in lite in the three Arabidopsis lines. Later on the change from darkness (0 μmol photon m–two s–ane) to loftier lite, m south consecration was initially faster in ST-OX and epf1 than WT during photosynthetic induction, while it was slower in ST-OX and epf1 than WT at the afterwards stage (Figure 3A). g due south in WT and epf1 was fully induced at 80 min after step increase in light, while that of ST-OX slightly only continuously increased in 120 min (Figure 2A). t 5 g s in ST-OX and epf1 was significantly shorter than that in WT (p < 0.05) (Figure 3B). On the other hand, t 60 g s in ST-OX and t 80 m s in ST-OX and epf1 were significantly larger than that in WT (p < 0.05). A induction was faster in ST-OX and epf1 than WT after the step increase in calorie-free (Figure 3C). t 60 A in ST-OX and epf1 and t fourscore A in epf1were significantly shorter than that in WT (p < 0.05) (Figure 3D). In the steady state under darkness, one thousand si in ST-OX and epf1 were 264.5% (p < 0.01) and 160.6% higher (non meaning), respectively, than that in WT (Figure 3E). t sixty A decreased with the increase in g si when g si < 0.074, and it was constantly independent of thou si for g si > 0.074 (Effigy 3F).
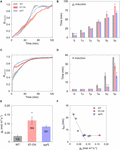
Figure three. The induction speed of stomatal conductance and CO2 assimilation rate after step increment in light. The induction state of (A) stomatal conductance (g southward ) and (C) CO2 assimilation charge per unit (A) were evaluated in the three lines of Arabidopsis based on 1000 sinduction and A induction defined as Eqs. 1 and 2, respectively, under a PPFD of 500 μmol photon chiliad–2 s–1 for 120 min after the dark flow for 10 min. The fourth dimension when (B) g s induction and (D) A induction reached 5, 10, 20, forty, 60, and 80% (t 5 – viii 0 g south and t 5 – 8 0 A ) of those maximum values was compared between WT and each transgenic line. (E) The steady-country value of chiliad s under the dark condition (one thousand s i ) was compared between WT and each transgenic line. (F) The human relationship was investigated between one thousand s i and t 60 A . Vertical confined indicate the standard error (n = 3). *indicates significant differences in each parameter between WT and each transgenic line at p < 0.05, according to Dunnett'south test. The values in each column stand for the relative value of each line to WT.
COtwo Assimilation and Biomass Product Under Fluctuating Light
Cumulative COii absorption and transpiration were evaluated to compare the efficiency of carbon gain and h2o utilise during photosynthetic induction in the three Arabidopsis lines. CCA in ST-OX and epf1 was 57.6 and 78.8% higher (p < 0.05), respectively, than that in WT, while Loss rate in ST-OX and epf1 was 27.seven% and 36.5% lower (p < 0.05) (Figures 4A,C). CE in ST-OX and epf1 were 193.7% and 138.7% higher (p < 0.05), respectively, than that in WT (Figure 4B). In that location was no significant variation in WUE i between WT and epf1, while WUE i in ST-OX was 44.9% lower than WT (p < 0.05) (Figure 4D). Finally, nosotros evaluated the biomass production under the constant (Figure 5A) and fluctuating low-cal (Figure 5B) in the three Arabidopsis lines to examine how higher SD affects growth characteristics. Compared with WT, dry out weight of the above basis biomass nether constant calorie-free (DW constant ) in epf1 was similar, while that under fluctuating light (DW fluctuating ) in epf1 was 25.half-dozen% higher than that of WT (p < 0.01) (Figures 5C,D). There was no significant variation in DW constant and DW fluctuating between ST-OX and WT.
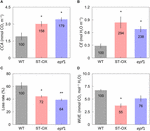
Effigy iv. Cumulative carbon proceeds and water use after stride increase in lite. (A) Cumulative CO2 assimilation (CCA) and (B) transpiration (CE) were measured in the first 10 min nether illumination after initial darkness in the three lines of Arabidopsis under the fluctuating low-cal. (C) The loss charge per unit of CO2 assimilation caused by the induction response was calculated based on Eq. 3. (D) Integrated water use efficiency (WUE i ) was calculated as the ratio of CCA to CE. Vertical bars betoken the standard error (n = three). * and ** indicate significant differences in each parameter betwixt WT and each transgenic line at p < 0.05 and 0.01, respectively, co-ordinate to Dunnett's test. The value in each cavalcade represents the relative value of each line to WT.
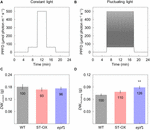
Figure 5. Biomass production under the constant and fluctuating low-cal conditions. Dry weight of the above footing biomass was evaluated in the three lines of Arabidopsis under (C) the constant (DW constant ) and (D) fluctuating (DW fluctuating ) low-cal conditions as described in (A) and (B). Vertical bars point the standard error (northward = four). ** indicates meaning differences in each parameter betwixt WT and each transgenic line at p < 0.01, according to Dunnett's examination. The values in each column stand for the relative value of each line to WT.
Discussion
Stomata play a meaning role in the regulation of gas commutation between the exterior and inside of the leaf. However, how the SD change affects photosynthetic and growth characteristics in plants has been controversial, and the effect of SD change on photosynthesis and growth can vary depending on the plant species or environmental conditions. Previously, we reported that college SD resulted in the enhancement of one thousand s and A in Arabidopsis under abiding and saturated light conditions (Tanaka et al., 2013). Lawson and Blatt (2014) suggested that with higher SD, it would exist instructive to determine biomass productivity nether fluctuating light, although only a few studies investigated the relationship between SD and photosynthetic or growth characteristics nether that condition (Drake et al., 2013; Papanatsiou et al., 2016; Schuler et al., 2017; Vialet-Chabrand et al., 2017). Hither, we attempted to examine how higher SD affects one thousand south and A dynamics, biomass production, and h2o use in Arabidopsis under fluctuating light.
Stomatal Density Affects the Induction of Stomatal Opening
We revealed that the three Arabidopsis lines differing in SD showed pregnant differences in the dynamics of chiliad due south in the not-steady state. SD differences had meaning (Vialet-Chabrand et al., 2017) or non-significant (Papanatsiou et al., 2016; Schuler et al., 2017) effect on g south induction to lite transients from low to high in previous studies. In the nowadays written report, ST-OX and epf1 showed initially faster grand south induction than WT, while those lines showed slower grand southward induction in the later on stage later on stride increase in light from a PPFD of 0 to 500 μmol photon m–two s–1 (Figures 3A,B). The different responses of yard southward could be attributable to the departure in the size, density, and patterning of stomata. Drake et al. (2013) reported that smaller stomata reply the fluctuating light faster than larger stomata amid several species of the genus Banksia. On the contrary, smaller stomata resulted in the slower response of chiliad due south to fluctuating calorie-free in the genus Oryza (Zhang et al., 2019). In the present written report, the variation in the speed of g s consecration did not represent to that in L m (Figures 1, iii), indicating that the stomatal size would have a minor result on g s induction in Arabidopsis under fluctuating low-cal.
The stomatal opening is regulated by at to the lowest degree three key components, blue-lite receptor phototropin, plasma membrane H+-ATPase, and plasma membrane inward rectifying Chiliad+ channels in the guard prison cell (Inoue and Kinoshita, 2017). The activation of H+-ATPase induced by blue light as the initial signal facilitates Thousand+ uptake through the inward rectifying K+ aqueduct to increase the turgor pressure level of guard cells, resulting in the stomatal opening. In add-on, stomatal opening dynamics depend on the water status in the plant (Lawson and Blatt, 2014). With more stomata, higher metabolic cost and water uptake would be required for stomatal movement. The gas-exchange and theoretical-modeling analysis indicated that the stomatal clustering decreased the maximum value of g s and A under the steady state because of the misplacement of stomatal pores over mesophyll cells (Dow and Bergmann, 2014; Lehmann and Or, 2015). It was also shown that clustering suppressed stomatal movement owing to the decreased capacity of the Thousand+ flux and K+ accumulation in the guard cells (Papanatsiou et al., 2016). Additionally, yard southward consecration to fluctuating lite in Begonia species with amassed stomata was slower than that in those without clustered stomata (Papanatsiou et al., 2017). In this report, ST-OX and epf1 with higher SD and clustering rate showed initially faster g s induction and, at the later phase, slower induction than WT (Figures 1, 3). These results suggest that the changes in stomatal density and patterning can affect thou s induction to fluctuating light owing to the alter in water uptake for stomatal opening and the opening speed of single stomata.
Stomatal Density Affects the Dynamics of COii Assimilation
In ST-OX and epf1, A induction to step alter from darkness to loftier light was faster than that of WT (Figures 3C,D). Photosynthetic consecration is typically limited by three phases of the biochemical or diffusional processes; (one) activation of electron transport, (ii) activation of the enzymes of the Calvin-Benson bicycle, and (3) stomatal opening (Pearcy, 1990; Yamori et al., 2016). The significance of stomatal limitation to photosynthetic induction depends on the initial value of yard s also equally photosynthetic chapters and the induction state of biochemical processes (Kirschbaum and Pearcy, 1988). Activation speed of the electron transport and enzymes of the Calvin-Benson bicycle after footstep increment in calorie-free intensity tin can be largely affected by COtwo concentration (Jackson et al., 1991; Urban et al., 2008; Kaiser et al., 2017). The variation of g s under dark or low calorie-free conditions corresponded to that in the speed of photosynthetic induction in several plant species (Kaiser et al., 2016; Soleh et al., 2017). In this report, t 60 A correlated with m si if yard si < 0.074 mol one thousand–two s–one, and it was constant regardless of yard si if g si > 0.074 mol m–2 southward–1 (Effigy 3F). 1000 s i of WT, ST-OX, and epf1 were 0.032, 0.118, and 0.085 mol g–2 s–ane, respectively (Figure 3E), suggesting that the variation in g s i would crusade the response difference of A. Therefore, higher SD resulted in higher initial value of g s and and then college C i , which would contribute to the rapid activation of RuBP regeneration and carboxylation in the Calvin-Benson bicycle.
The transition from a short period of low to high light is frequently observed in the ingather canopy throughout the day (Tanaka et al., 2019). The nowadays written report confirmed that higher SD resulted in faster A consecration later on step increment in calorie-free from darkness, which tin can exist observed at the limited part of the day in field. It is not clear how SD affects g s and A induction after the accommodation to low light for short menses. It has been considered that stomatal opening and Rubisco activation would not be a major limitation to A under such calorie-free conditions since these would non modify rapidly (MuAusland et al., 2016). A rapid change in the RuBP regeneration was reported to limit photosynthetic induction nether high light after a short catamenia of low light or darkness (Kobza and Edwards, 1987; Sassenrath-Cole and Pearcy, 1994). On the other hand, the significant stomatal limitation to photosynthesis has been shown in Arabidopsis (Kimura et al., 2020) and rice (Yamori et al., 2020) nether natural light weather condition where the light fluctuations are highly variable. Futurity study is required to elucidate that higher SD would be beneficial for carbon proceeds under more rapid and frequent fluctuation of light.
Stomatal Density Affects Biomass Production Nether the Fluctuating Light
Manipulating COii improvidence via stomata has been attempted to enhance photosynthetic capacity and induction in plants. Under constant low-cal atmospheric condition, overexpression of H+-ATPase (AHA2) in guard cells resulted in higher m south as well as A, leading to greater biomass production in Arabidopsis (Wang et al., 2014). In addition, Arabidopsis plants with stay-opening or fast-moving stomata have been shown to accomplish greater carbon gain and biomass production nether fluctuating low-cal conditions (Papanatsiou et al., 2019; Kimura et al., 2020). These studies confirmed the significant limitation of photosynthesis imposed past stomata, and the potential of g southward to improve biomass production of plants under field. On the other hand, higher thou s by and large results in lower WUE, which can depress the benefit of greater photosynthetic operation for biomass production (Tanaka et al., 2013; Kimura et al., 2020). Under drought condition, transgenic plants with lower SD and g south exhibited improved growth operation owing to high WUE in several species (Yoo et al., 2010; Wang et al., 2016; Caine et al., 2018). It is, therefore, import to optimize a residual between carbon gain and water loss via stomata for plant growth depending on h2o weather condition (Lawson and Blatt, 2014; MuAusland et al., 2016).
DW fluctuating was much lower than DW abiding in three Arabidopsis lines, although the total amount of light intensity exposed to the plants was equal betwixt both calorie-free conditions (Effigy 5). This departure would exist caused past the loss of carbon gain owing to photosynthetic induction under fluctuating light condition. DW abiding in ST-OX was slightly lower than that in WT, although steady-state A was significantly or slightly college in Tanaka et al. (2013) and this study, respectively (Figures 2, v). The increment in h2o loss would have a negative effect on biomass production in ST-OX under constant light (Tanaka et al., 2013). ST-OX showed significantly lower WUE during photosynthetic consecration in the present study (Figures ii, 4). Despite of these penalties resulting from the drastic increment in SD, DW fluctuating in ST-OX was 10.5% higher than that in WT with no significance. Moreover, biomass production in epf1, with moderate increase in SD, was significantly higher than that in WT under fluctuating light, while there was no deviation betwixt these ii lines under abiding light (Effigy five). It is possible that a moderate increase in SD could achieve more than efficient carbon gain attributable to the faster response of A in Arabidopsis under fluctuating light, while information technology would cause small penalties on h2o loss for stomatal motion. Overall, higher SD can be beneficial to improve biomass production in plants under fluctuating calorie-free weather under favorable water conditions.
Conclusion
Under fluctuating light, there was a significant variation in the photosynthetic and growth characteristics amidst Arabidopsis lines differing in the stomatal density (SD). Higher SD resulted in faster COtwo absorption charge per unit (A) induction to fluctuating light owing to the higher initial value of the stomatal conductance (g southward ) and faster yard south induction in the early phase of photosynthetic induction. On the other hand, higher SD resulted in slower thou s induction in the subsequently phase of photosynthetic induction. epf1, with a moderate increase in SD, achieved more efficient carbon gain with modest penalty on h2o use efficiency owing to the faster A induction, which would contribute to higher biomass production than that in WT under fluctuating lite. This study suggests that higher SD tin can exist beneficial to ameliorate biomass product in plants nether fluctuating light.
Data Availability Statement
The original contributions presented in the written report are included in the commodity/Supplementary Textile, further inquiries can be directed to the corresponding author.
Author Contributions
KS conceived and designed this projection, performed all the gas substitution experiments, wrote the manuscript with inputs from co-authors. WY conducted the biomass assay. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a Grant-in-Aid for Scientific Research to IH-N (Grant Nos. 22000014 and 15H05776), to KS (Grant No. 20J00594), to WY (Grant Nos. 16H06552, 18H02185, 18KK0170, and 20H05687), and to YT (Grant No. 20H02968) from Japan Society for the Promotion of Science (JSPS), and PRESTO to YT (Grant No. JPMJPR16Q5) from Nippon Science and Technology Agencys.
Conflict of Interest
The authors declare that the research was conducted in the absence of whatever commercial or financial relationships that could exist construed as a potential disharmonize of interest.
Acknowledgments
Nosotros are grateful to Mr. S. Shimadzu for help in the biomass analysis. This manuscript has been released as a pre-print at bioRxiv, Sakoda et al. (2020).
Supplementary Material
The Supplementary Material for this article tin be constitute online at: https://world wide web.frontiersin.org/manufactures/10.3389/fpls.2020.589603/full#supplementary-textile
Supplementary Effigy 1 | Stomatal conductance and CO2 assimilation rate under steady state. (A) A stomatal conductance (g sf ) and (B) CO2 assimilation rate (A f ) nether steady state were measured on fully expanded leaves in the three lines of Arabidopsis. The gas commutation measurements were conducted at a COii concentration of 400 ppm, air temperature of 25°C and night condition for the initial 10 min and, subsequently, under a PPFD of 500 μmol photon m–2 south–one for 120 min. Vertical bars signal the standard error (n = 3). The values in each column stand for the relative value of each line to WT.
References
Adachi, S., Tanaka, Y., Miyagi, A., Kashima, M., Tezuka, A., Toya, Y., et al. (2019). High-yielding rice Takanari has superior photosynthetic response under fluctuating lite to a commercial rice Koshihikari. J. Exp. Bot. 70, 5287–5297. doi: x.1093/jxb/erz304
PubMed Abstract | CrossRef Full Text | Google Scholar
Büssis, D., Von Groll, U., Fisahn, J., and Altmann, T. (2006). Stomatal aperture can compensate altered stomatal density in Arabidopsis thaliana at growth light conditions. Funct. Plant Biol. 33, 1037–1043. doi: 10.1071/FP06078
PubMed Abstract | CrossRef Total Text | Google Scholar
Caine, R., Yin, Ten., Sloan, J., Harrison, East. L., Mohammed, U., Fulton, T., et al. (2018). Rice with reduced stomatal density conserves water and has improved drought tolerance nether future climate conditions. New Phytol. 221, 371–384. doi: 10.1111/nph.15344
PubMed Abstract | CrossRef Full Text | Google Scholar
Carmo-Silva, A. East., and Salvucci, Thou. E. (2013). The regulatory properties of rubisco activase differ among species and affect photosynthetic induction during calorie-free transitions. Institute Physiol. 161, 1645–1655. doi: 10.1104/pp.112.213348
PubMed Abstruse | CrossRef Full Text | Google Scholar
Doheny-Adams, T., Hunt, L., Franks, P. J., Beerling, D. J., and Gray, J. E. (2012). Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc. B Biol. Sci. 367, 547–555. doi: 10.1098/rstb.2011.0272
PubMed Abstract | CrossRef Full Text | Google Scholar
Dow, G. J., and Bergmann, D. C. (2014). Patterning and processes: how stomatal development defines physiological potential. Curr. Opin. Constitute Biol. 21, 67–74. doi: ten.1016/j.pbi.2014.06.007
PubMed Abstract | CrossRef Full Text | Google Scholar
Drake, P. L., Froend, R. H., and Franks, P. J. (2013). Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 64, 495–505. doi: 10.1093/jxb/ers347
PubMed Abstract | CrossRef Full Text | Google Scholar
Farquhar, G. D., and Sharkey, T. D. (1982). Stomatal conductance and photosynthesis. Annu. Rev. Institute Physiol. 33, 317–345. doi: 10.1146/annurev.pp.33.060182.001533
CrossRef Full Text | Google Scholar
Franks, P. J., and Beerling, D. J. (2009). CO2-forced evolution of plant gas substitution chapters and water-use efficiency over the phanerozoic. Geobiology 7, 227–236. doi: 10.1111/j.1472-4669.2009.00193.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Hara, K., Kajita, R., Torii, K. U., Bergmann, D. C., and Kakimoto, T. (2007). The secretory peptide gene EPF1. Genes Dev. 7, 1720–1725. doi: ten.1101/gad.1550707.metric
CrossRef Full Text | Google Scholar
Hashimoto-Sugimoto, Chiliad., Higaki, T., Yaeno, T., Nagami, A., Irie, One thousand., Fujimi, G., et al. (2013). A Munc13-like protein in Arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nat. Commun. 4:3215. doi: 10.1038/ncomms3215
PubMed Abstruse | CrossRef Full Text | Google Scholar
Jackson, R. B., Woodrow, I. E., and Mott, K. A. (1991). Nonsteady-country photosynthesis post-obit an increment in photon flux density (PFD). Found Physiol. 95, 498–503. doi: 10.1104/pp.95.2.498
PubMed Abstruse | CrossRef Full Text | Google Scholar
Kaiser, East., Kromdijk, J., Harbinson, J., Heuvelink, E., and Marcelis, L. F. M. (2017). Photosynthetic consecration and its diffusional, carboxylation and electron send processes as affected past COtwo fractional pressure, temperature, air humidity and blue irradiance. Ann. Bot. 119, 191–205. doi: x.1093/aob/mcw226
PubMed Abstract | CrossRef Full Text | Google Scholar
Kaiser, E., Morales, A., Harbinson, J., Heuvelink, E., Prinzenberg, A. Due east., and Marcelis, 50. F. One thousand. (2016). Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Sci. Rep. six, 1–13. doi: 10.1038/srep31252
PubMed Abstract | CrossRef Full Text | Google Scholar
Kimura, H., Hashimoto-Sugimoto, K., Iba, K., Terashima, I., and Yamori, W. (2020). Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating lite. J. Exp. Bot. 71, 2339–2350. doi: 10.1093/jxb/eraa090
PubMed Abstract | CrossRef Total Text | Google Scholar
Kirschbaum, M. U. F., and Pearcy, R. Westward. (1988). Gas exchange analysis of the fast phase of photosynthetic consecration in Alocasia macrorrhiza. Plant Physiol. 87, 818–821. doi: 10.1104/pp.87.4.818
PubMed Abstract | CrossRef Full Text | Google Scholar
Kobza, J., and Edwards, M. East. (1987). The photosynthetic induction response in wheat leaves: net CO2 uptake, enzyme activation, and leaf metabolites. Planta 171, 549–559. doi: 10.1007/BF00392305
PubMed Abstract | CrossRef Total Text | Google Scholar
Lawson, T., and Blatt, M. R. (2014). Stomatal size, speed, and responsiveness bear upon on photosynthesis and water use efficiency. Plant Physiol. 164, 1556–1570. doi: x.1104/pp.114.237107
PubMed Abstract | CrossRef Full Text | Google Scholar
Lee, J. S., Hnilova, M., Maes, K., Lin, Y. C. L., Putarjunan, A., Han, S. One thousand., et al. (2015). Competitive bounden of antagonistic peptides fine-tunes stomatal patterning. Nature 522, 439–443. doi: 10.1038/nature14561
PubMed Abstract | CrossRef Full Text | Google Scholar
MuAusland, L., Vialet-Chanbrand, S., Davey, P., Bakery, Due north. R., Brendel, O., and Lawson, T. (2016). Effects of kinetics of calorie-free-induced stomatal responses on photosynthesis and water-employ efficiency. New Phytol. 211, 1209–1220. doi: 10.1111/nph.14000
PubMed Abstract | CrossRef Full Text | Google Scholar
Papanatsiou, Yard., Amtmann, A., and Blatt, Yard. R. (2016). Stomatal spacing safeguards stomatal dynamics by facilitating guard cell ion transport contained of the epidermal solute reservoir. Found Physiol. 172, 254–263. doi: 10.1104/pp.16.00850
PubMed Abstract | CrossRef Full Text | Google Scholar
Papanatsiou, Thousand., Amtmann, A., and Blatt, M. R. (2017). Stomatal clustering in Begonia associates with the kinetics of foliage gaseous commutation and influences h2o employ efficiency. J. Exp. Bot. 68, 2309–2315. doi: 10.1093/jxb/erx072
PubMed Abstract | CrossRef Full Text | Google Scholar
Papanatsiou, M., Petersen, J., Henderson, Fifty., Wang, Y., Christie, J. M., and Blatt, Thousand. R. (2019). Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water utilize, and growth. Scientific discipline 363, 1456–1459. doi: 10.1126/scientific discipline.aaw0046
PubMed Abstract | CrossRef Full Text | Google Scholar
Pearcy, R. W. (1990). Sunflecks and photosynthesis in establish canopies. Ann. Rev. Plant Biol. 41, 421–453. doi: 10.1146/annurev.pp.41.060190.002225
CrossRef Full Text | Google Scholar
Qu, M., Hamdani, S., Li, W., Wang, S., Tang, J., Chen, Z., et al. (2016). Rapid stomatal response to fluctuating light: an under-explored machinery to improve drought tolerance in rice. Funct. Plant Biol. 43:727. doi: ten.1071/fp15348
PubMed Abstract | CrossRef Full Text | Google Scholar
Sakoda, Chiliad., Kaga, One thousand., Tanaka, Y., Suzuki, Southward., Fujii, K., Ishimoto, One thousand., et al. (2018). Two novel quantitative trait loci affecting the variation in leaf photosynthetic chapters among soybeans. Plant Sci. 291:110300. doi: 10.1016/j.plantsci.2019.110300
PubMed Abstract | CrossRef Total Text | Google Scholar
Sakoda, K., Yamori, W., Shimada, T., Sugano, S. Southward., Hara-Nishimura, I., and Tanaka, Y. (2020). Stomatal density affects gas diffusion and COii assimilation dynamics in Arabidopsis nether fluctuating light. bioRxiv [Preprint]. doi: x.1101/2020.02.20.958603
CrossRef Total Text | Google Scholar
Sassenrath-Cole, G. F., and Pearcy, R. W. (1994). Regulation of photosynthetic induction land past the magnitude and duration of low light exposure. Plant Physiol. 105, 1115–1123. doi: 10.1016/j.biopsych.2016.03.2100
PubMed Abstract | CrossRef Full Text | Google Scholar
Schlüter, U., Muschak, M., Berger, D., and Altmann, T. (2003). Photosynthetic performance of an Arabidopsis mutant with elevated stomatal density (sdd1-one) under different light regimes. J. Exp. Bot. 54, 867–874. doi: 10.1093/jxb/erg087
PubMed Abstract | CrossRef Full Text | Google Scholar
Schuler, M. L., Sedelnikova, O. 5., Walker, B. J., Westhoff, P., and Langdale, J. A. (2017). SHORTROOT-mediated increase in stomatal density has no impact on photosynthetic efficiency. Plant Physiol. 176, 752–772. doi: x.1104/pp.17.01005
PubMed Abstract | CrossRef Full Text | Google Scholar
Shimadzu, South., Seo, Thousand., Terashima, I., and Yamori, W. (2019). Whole irradiated plant leaves showed faster photosynthetic induction than individually irradiated leaves via improved stomatal opening. Front. Institute Sci. 10:1512. doi: 10.3389/fpls.2019.01512
PubMed Abstruse | CrossRef Full Text | Google Scholar
Soleh, One thousand. A., Tanaka, Y., Kim, S. Y., Huber, Due south. C., Sakoda, K., and Shiraiwa, T. (2017). Identification of large variation in the photosynthetic induction response amid 37 soybean [Glycine max (L.) Merr.] genotypes that is not correlated with steady-land photosynthetic capacity. Photosynth. Res. 131, 305–315. doi: 10.1007/s11120-016-0323-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Soleh, M. A., Tanaka, Y., Nomoto, Y., Iwahashi, Y., Nakashima, M., Fukuda, Y., et al. (2016). Factors underlying genotypic differences in the induction of photosynthesis in soybean [Glycine max (L.) Merr.]. Plant Jail cell Environ. 39, 685–693. doi: ten.1111/pce.12674
PubMed Abstruse | CrossRef Full Text | Google Scholar
Sugano, Southward. S., Shimada, T., Imai, Y., Okawa, K., Tamai, A., Mori, M., et al. (2010). Stomagen positively regulates stomatal density in Arabidopsis. Nature 463, 241–244. doi: ten.1038/nature08682
PubMed Abstract | CrossRef Full Text | Google Scholar
Tanaka, Y., Adachi, Southward., and Yamori, W. (2019). Natural genetic variation of the photosynthetic induction response to fluctuating lite environment. Curr. Opin. Plant Biol. 49, 52–59. doi: 10.1016/j.pbi.2019.04.010
PubMed Abstract | CrossRef Full Text | Google Scholar
Tanaka, Y., Sugano, Due south. S., Shimada, T., and Hara-Nishimura, I. (2013). Enhancement of foliage photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 198, 757–764. doi: 10.1111/nph.12186
PubMed Abstruse | CrossRef Full Text | Google Scholar
Taylor, S. H., and Long, S. P. (2017). Dull induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity. Philos. Trans. R. Soc. B Biol. Sci. 17:372. doi: 10.1098/rstb.2016.0543
PubMed Abstract | CrossRef Full Text | Google Scholar
Urban, O., Šprtová, M., Košvancová, M., Tomášková, I., Lichtenthaler, H. Yard., and Marek, One thousand. V. (2008). Comparison of photosynthetic induction and transient limitations during the induction phase in immature and mature leaves from 3 poplar clones. Tree Physiol. 28, 1189–1197. doi: 10.1093/treephys/28.8.1189
PubMed Abstruse | CrossRef Total Text | Google Scholar
Vialet-Chabrand, S. R. M., Matthews, J. Southward. A., McAusland, 50., Blatt, Grand. R., Griffiths, H., and Lawson, T. (2017). Temporal dynamics of stomatal behavior: modeling and implications for photosynthesis and water use. Plant Physiol. 174, 603–613. doi: 10.1104/pp.17.00125
PubMed Abstract | CrossRef Total Text | Google Scholar
Wang, C., Liu, S., Dong, Y., Zhao, Y., Geng, A., Xia, X., et al. (2016). PdEPF1 regulates water-use efficiency and drought tolerance by modulating stomatal density in poplar. Establish Biotechnol. J. xiv, 849–860. doi: ten.1111/pbi.12434
PubMed Abstract | CrossRef Total Text | Google Scholar
Wang, Y., Noguchi, K., Ono, N., Inoue, South., Terashima, I., and Kinoshita, T. (2014). Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc. Natl. Acad. Sci. U.s.A. 111, 533–538. doi: 10.1073/pnas.1305438111
PubMed Abstract | CrossRef Total Text | Google Scholar
Wong, S. C., Cowan, I. R., and Farquhar, G. D. (1979). Stomatal conductance correlates with photosynthetic capacity. Nature 282, 424–426. doi: 10.1038/282424a0
CrossRef Total Text | Google Scholar
Yamori, West. (2016). Photosynthetic response to fluctuating environments and photoprotective strategies under abiotic stress. J. Plant Res. 129, 379–395. doi: 10.1007/s10265-016-0816-1
PubMed Abstract | CrossRef Full Text | Google Scholar
Yamori, W., Kondo, Eastward., Sugiura, D., Terashima, I., Suzuki, Y., and Makino, A. (2016). Enhanced foliage photosynthesis as a target to increment grain yield: Insights from transgenic rice lines with variable Rieske FeS protein content in the cytochrome b6/f complex. Institute Cell Environ. 39, 80–87. doi: x.1111/pce.12594
PubMed Abstract | CrossRef Full Text | Google Scholar
Yamori, W., Kusumi, K., Iba, K., and Terashima, I. (2020). Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating calorie-free conditions in rice. Plant. Prison cell Environ. 43, 1230–1240. doi: 10.1111/pce.13725
PubMed Abstract | CrossRef Full Text | Google Scholar
Yamori, W., Masumoto, C., Fukayama, H., and Makino, A. (2012). Rubisco activase is a cardinal regulator of non-steady-state photosynthesis at any leaf temperature and, to a bottom extent, of steady-state photosynthesis at high temperature. Plant J. 71, 871–880. doi: 10.1111/j.1365-313X.2012.05041.x
PubMed Abstract | CrossRef Full Text | Google Scholar
Yoo, C. Y., Pence, H. E., Jin, J. B., Miura, K., Gosney, 1000. J., Hasegawa, P. M., et al. (2010). The Arabidopsis GTL1 transcription cistron regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22, 4128–4141. doi: ten.1105/tpc.110.078691
PubMed Abstruse | CrossRef Full Text | Google Scholar
Zhang, Q., Peng, South., and Li, Y. (2019). Increase charge per unit of light-induced stomatal conductance is related to stomatal size in the genus Oryza. J. Exp. Bot. lxx, 5259–5269. doi: x.1093/jxb/erz267
PubMed Abstruse | CrossRef Full Text | Google Scholar
christiannoing1976.blogspot.com
Source: https://www.frontiersin.org/articles/10.3389/fpls.2020.589603/full
0 Response to "What Will Happen to the Rate of Photosynthesis if the Size of the Stomata Is Increased?"
Post a Comment